Matter
What's the Matter?
What do Justin Bieber, a pickle, and your pet gerbil have in common?They're all matter.
Matter is physically present stuff. It is tangible and takes up space. Your phone is made up of matter, as are raindrops, roses, and whiskers on kittens. Even tiny things like protons, neutrons, atoms, and molecules are matter because they have mass and volume. Mass is the weight of matter usually expressed in grams (g). Volume is the measure of how much space a substance occupies and is usually expressed in liters (L) or milliliters (mL).
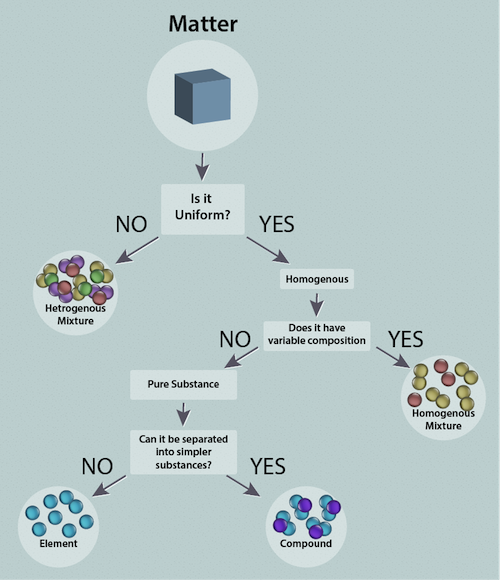
A collection of matter with uniform properties throughout, consisting of a single phase, is said to be homogeneous. What's a phase? We're glad you asked. A phase is a sample of matter that is uniform throughout both in its chemical composition and in its physical state. Confused? Don't worry—we'll go into more detail later. Examples include a glass of water, air, or a lump of coal. The presence of more than one distinct phase makes a collection of matter heterogeneous. Think of that same glass of water. Now add ice. The mixture is now heterogeneous.


Examples of homogeneous mixtures: glass of water (left) and an emerald (right). Image from here.

Example of a heterogeneous mixture: ice water.
Are you confused about the glass of milk example? This has to be a mistake, right? Wrong. To the naked eye, milk appears to be single phase, but if we look at a sample of milk under a microscope you would observe little globs of solid fat amongst the liquid particles. Those globs of fat are so small they float in the liquid milk forming a suspension, never sinking to the bottom.
Remember Little Miss Muffet? We know you know it. "Little Miss Muffet sat on a tuffet, eating her curds and whey…" Ever wonder exactly what she was eating? Curds and whey are the solids and liquids that result after milk is allowed to separate, usually with the help of a coagulant that lumps the solids together. Fascinated? Perhaps you should become a cheese maker.

Milk separated into curds and whey. Image from here.
When we say mixture, we mean a collection of matter that can be separated into two (or more) substances by physical means. Our glass of ice water is a mixture because we can easily separate the ice from the liquid water by filtration. If a homogeneous material cannot be separated into different components, then it is called a substance. We can further classify substances into compounds and elements. If a substance can be broken down, or decomposed, into simpler substances by a chemical process (anything that makes or breaks bonds) we call it a compound. If it cannot be broken down we call it an element. Consider sodium chloride (NaCl). If the Na-Cl bond is broken we have an Na atom and a Cl atom, therefore NaCl is an compound, while the Na and Cl atoms are elements because they cannot be broken down further.
Here's something to think about. How do we classify salt water? That depends. If we have a glass of water and dump in a bunch of salt, we would have a heterogeneous mixture because there is liquid water and solid salt. If we stir or heat that mixture until all of the salt dissolves you would have a homogenous mixture because there is one single phase and the entire solution is the same throughout.