Think you’ve got your head wrapped around Thermodynamics? Put your knowledge to
the test. Good luck — the Stickman is counting on you!
Q. During a phase change, ______________.
all the heat goes into changing the phase of matter and there is no change in temperature
all of the heat goes into raising or dropping the temperature of matter so that it can experience a phase change
the temperature of matter crosses the boiling or freezing point
how much the temperature of matter increases depends on the latent heat of fusion or vaporization
the temperature always increases
Q. What is the internal energy of 5 moles of an ideal gas at a temperature of 50 °C?
20131 J
1558 J
6.69 × 10-21 J
5.18 × 10-22 J
250 J
Q. Which of the following is not an assumption made by the kinetic theory of gases?
The gas is made up of a very large number of molecules.
The molecules are very far apart from each other.
The molecules lose energy when they interact with each other or with the walls of the container.
The molecules all have the same mass.
The molecules move randomly.
Q. Which of the following statements about heat engines is correct?
All of the heat energy in a heat engine can be turned into mechanical work.
The most efficient heat engines are irreversible.
A heat engine cannot have a Carnot efficiency below 1.
A heat engine can only have a Carnot efficiency of 1 if the temperature of the heat reservoir and the temperature of the cold reservoir are the same.
A heat engine can only have a Carnot efficiency of 1 if the temperature of the cold reservoir is at absolute zero.
Q. A gas is at a temperature of 237 K, has a volume of 20 × 10-3 m3, and the pressure of the gas is 5 × 105 Pa. If the pressure doubles, and the volume of the gas stays the same, by how much does the temperature change?
474 K
402 K
402 °C
474 °C
119 K
Q. During isothermal expansion of a gas, ______________.
work is done on the system
work is done by the system
no work is done
the internal energy of the system does not change
the work done is equal to the change in the internal energy of the system
Q. The work done during adiabatic expansion of a gas depends on ______________.
the volume, pressure, and temperature of the gas
the change in temperature and the number of moles of gas
the temperature and the number of moles of gas
the pressure and the change in volume of the gas
the change in pressure and the change in volume of the gas
Q. The mass of a nitrogen molecule is 4.65 × 10-26 kg. On a hot summer day, when the temperature hits 40 °C, what is the root-mean-square velocity of nitrogen molecules in the air?
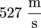



We need to know the atmospheric pressure
Q. There are 3 × 105 Joules of heat transferred into a 50 kg of water. The specific heat capacity of water is  . The same amount of heat is transferred into a 50 kg person. The specific heat capacity of the human body is
. The same amount of heat is transferred into a 50 kg person. The specific heat capacity of the human body is  . What is the difference in the increase in temperature between the water and the person?
. What is the difference in the increase in temperature between the water and the person?
 . The same amount of heat is transferred into a 50 kg person. The specific heat capacity of the human body is
. The same amount of heat is transferred into a 50 kg person. The specific heat capacity of the human body is  . What is the difference in the increase in temperature between the water and the person?
. What is the difference in the increase in temperature between the water and the person? The change in temperature is the same.
ΔTperson = 1.71 °C; ΔTwater = 1.43 °C
ΔTperson = 1.71 K; ΔTwater = 1.43 K
1.45 × 1028 K in both cases
Both b and c
Q. A heat engine draws 400 J from its hot reservoir and deposits 100 J into its cold reservoir. How much work is done by this engine?
300 J
500 J
400 J
100 J
Cannot be determined