ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
AP Physics 2: 2.1 Probability and Systems 171 Views
Share It!
Description:
AP Physics 2: 2.1 Probability and Systems. During which of the following sequences is the energy change equal to zero?
Transcript
- 00:00
Thank you We sneak and here's your shmoop you sure
- 00:05
Brought to you by pressure and volume there's a relationship
- 00:08
between pressure and volume As in when we eat three
- 00:11
cubic leaders of mac and cheese the pressure on our
- 00:13
stomach reaches well Epic proportions Take a look at this
Full Transcript
- 00:17
pressure volume diagram Right here Here we go The path
- 00:20
from point c to point a is an ice Oh
- 00:23
thermal expansion and the number of molecules is such that
- 00:26
the number of molecules times bowl timmons constant equals the
- 00:30
number of malls times the gas constant which also equals
- 00:33
one jule per kilogram During which of the following sequences
- 00:37
is the energy change equal to zero There you go
- 00:41
Pointing at point b b c c a and here's
- 00:44
a potential answers Yeah we're on the numeral time Yeah
- 00:47
That's what i got right So we're talking about a
- 00:49
change in energy here being a change in temperature The
- 00:52
equation for the ideal gas law is pressure times Volume
- 00:55
equals the number of moles times the gas constant times
- 00:58
temperature The number of moles in the gas constant will
- 01:01
stay the same here After all we're not taking any
- 01:03
gas away and the gas constant is well constant So
- 01:07
in order for the temperature not change in the product
- 01:09
on the left side of the equation has remained the
- 01:11
same Let's Look at this diagram again Moving from a
- 01:15
to b means the volume will change but the pressure
- 01:17
will stay the same and moving from b to c
- 01:20
means the pressure will change but the volume will remain
- 01:23
stable Neither of these scenarios the pv side of the
- 01:26
equation real result in a change of temperature But in
- 01:30
the change from c t es the pressure lowers the
- 01:32
volume increases The left side of the equation will remain
- 01:36
the same and the temperature energy won't change So the
- 01:39
correct answer is c in a pressure volume equation the
- 01:43
variables are dependent on each other So if one part
- 01:46
changes other parts will change too Just like if we
- 01:49
eat less our stomach pressure reduces as does our happiness 00:01:53.502 --> [endTime] But that's not a trade we're willing to make
Up Next
Related Videos
AP Physics 2: 1.1 Properties of Objects and Systems. What is the magnitude and direction of the conventional current in this wire?
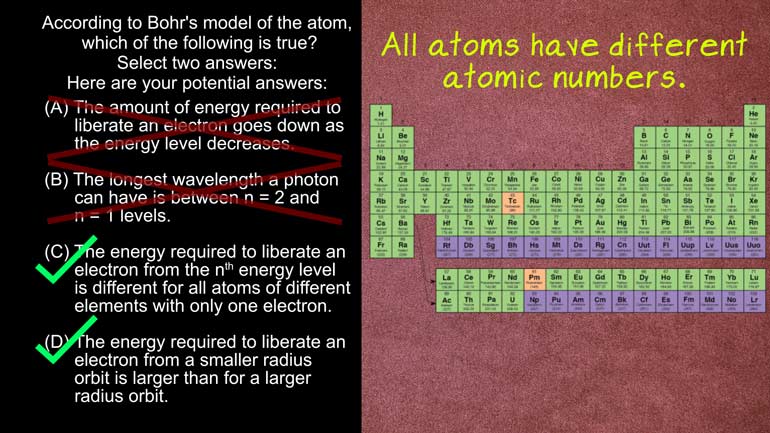
AP Physics 2: 1.5 Properties of Objects and Systems. According to the Bohr's model of the atom, which of the following are true?
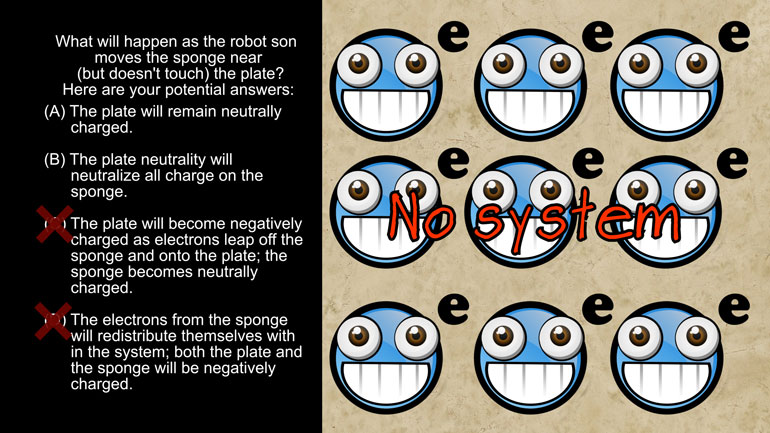
AP Physics 2: 2.2 Properties of Objects and Systems. What will happen as the robot son moves the sponge near (but doesn't touch) the plate?
AP Physics 2: 2.4 Properties of Objects and Systems. How could you show the carnival barker an emission spectrum?