ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
AP Chemistry Videos 45 videos
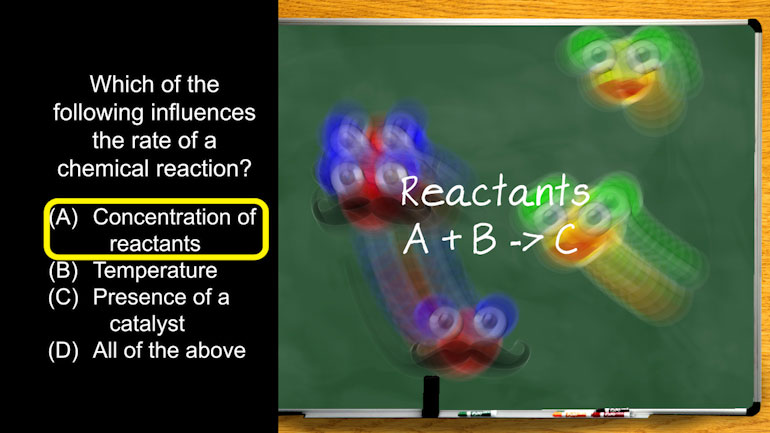
AP Chemistry 3.5 Chemical Reaction Rates. How much 85Kr will we need to collect?
Want to pull an Oliver Twist and ask us for more? We've gotcha covered. Head over to /video/subjects/test-prep/ap/ap-chemistry/ for more AP Ch...
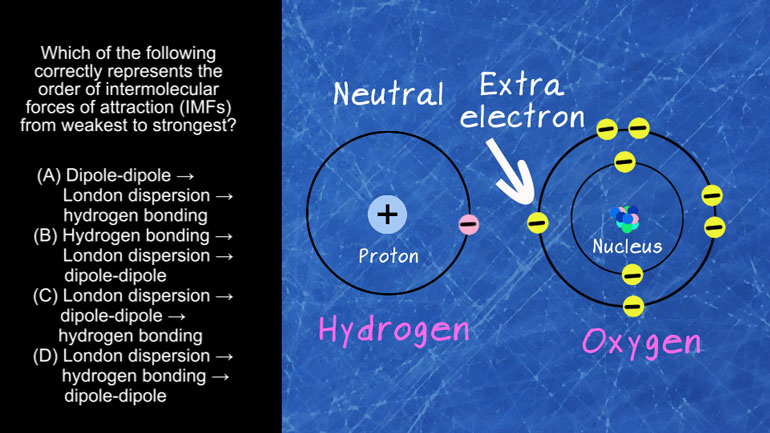
AP Chemistry 1.3 Forming and Breaking Bonds. Which of the following ions are spectator ions?
AP Chemistry 2.3 Structure and Arrangement of Atoms 27 Views
Share It!
Description:
AP Chemistry 2.3 Structure and Arrangement of Atoms. According to VSEPR theory, what is the geometry of the hybrid orbitals on carbon in the molecule represented by the Lewis structure above?
Transcript
- 00:04
Here’s your Shmoop du jour, brought to by bond.
- 00:07
Ionic bond. [James Bond taking glass of champagne]
- 00:08
Taken, not shared.
- 00:10
Consider the following molecule:
- 00:12
And here's our question…
Full Transcript
- 00:14
According to VSEPR theory, what is the geometry of the hybrid orbitals on carbon in the molecule
- 00:20
represented by the Lewis structure above?
- 00:24
Here are our potential answers…
- 00:28
So what is VSEPR?
- 00:30
Voracious spies eat pizza regularly? [Man eating pizza slice]
- 00:33
Very serious elephants pick radishes…. [Elephant eating radishes]
- 00:35
No, unfortunately it has nothing to do with cool spies and elephants.
- 00:39
VSEPR stands for Valence Shell Electron Pair Repulsion… [Valence and shell meet each other]
- 00:44
…which is just a fancy way of saying that electrons in a molecule would rather not hang
- 00:48
around each other.
- 00:49
So what this question is actually asking is “what shape will put the electrons in this
- 00:55
molecule as far away from each other as possible?”
- 00:59
The electrons that we're worried about are the ones in the bonds and lone pairs around [Electrons orbiting in shell]
- 01:03
the central atom.
- 01:04
The central atom is exactly what it sounds like-the atom at the creamy center of the
- 01:09
molecular candy bar. [Girl eating a lollipop]
- 01:13
To find it, we have to find the atom bonded to the highest number of other atoms.
- 01:17
In our molecule, the chlorines and oxygen have 1 other atom bonded to them and the carbon
- 01:22
has 3 other atoms bonded to it.
- 01:25
So carbon is the central atom of this molecule.
- 01:30
We then count the number of lone pairs of electrons and bonds on the carbon in the Lewis [Carbon with electron pairs]
- 01:34
dot structure.
- 01:35
And…quick count, here…
- 01:37
There are no lone pairs, which will make Valentine's Day much more tolerable this year… [Man and woman holding hands]
- 01:42
…there are 2 single bonds…which…eh, there's always the day after Valentine's candy
- 01:47
sale to look forward to…
- 01:48
…and there's 1 double bond, which acts the same as a single bond when determining shape.
- 01:53
What a player.
- 01:54
Now let’s figure out what shape puts these bonds equally far apart; we don’t want to [Examples of various bonds appear]
- 01:59
put two bonds far apart from each other just to find out we put them closer to the third
- 02:04
bond.
- 02:05
In order to figure this out, let’s pull out some dice. [Dice roll]
- 02:07
Dice are a good way to visualize shapes with the bonds as far away from each other as possible,
- 02:12
because dice have a regular geometry.
- 02:15
Tetra means four…no not Tetris-close your other tab…and Octa means eight. [Person playing tetris]
- 02:20
This means a tetrahedral shape is one with four sides and an ocatahedral shape has eight
- 02:26
sides.
- 02:27
If we look at our four sided die, it has four corners.
- 02:32
The number of corners on our die is the number of atoms bonded to the central atom of the
- 02:36
molecule that has that shape.
- 02:39
Our central carbon is bonded to 3 things, not four, so the shape is not tetrahedral. [electrons appear by central carbon]
- 02:44
Similarly, if we look at our eight sided die, we can count six corners.
- 02:50
This means the central atom will have six things bonded to it in an octahedral shape,
- 02:56
and our molecule only has three.
- 02:58
So our molecule isn't octahedral.
- 03:00
This rules out answers A and D Both trigonal planar and trigonal bipyramidal
- 03:05
look similar and have tri in the name which, as we remember [Girl riding a tricycle]
- 03:09
from our preschool days of tricycle riding, means three
- 03:13
However, trigonal BIpyramidal has bi in it, meaning two; there are actually two pyramids [Man riding a bicycle]
- 03:19
in the shape of this molecule.
- 03:21
A trigonal bipyramid shape involves bonds to five different atoms, which our carbon
- 03:25
doesn't have…
- 03:27
Therefore, the answer must be C. Trigonal Planar.
- 03:30
It's the most travelled airline of molecules [Trigonal planar aircraft flying]
- 03:33
everywhere.
Related Videos
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?
AP Chemistry 3.2 Laws of Thermodynamics. What is the value for ΔG?