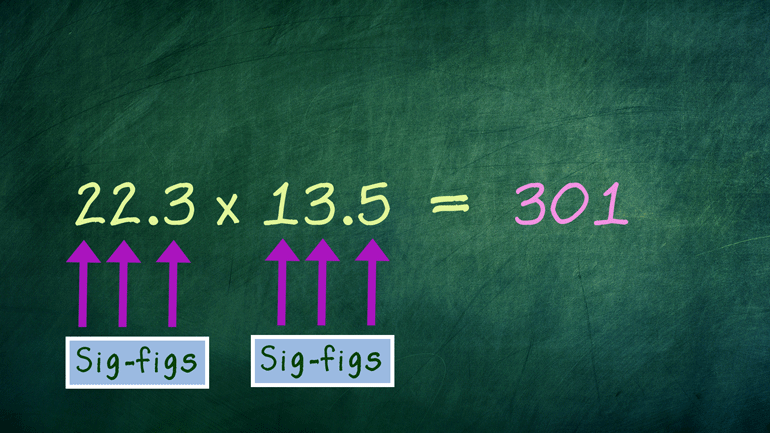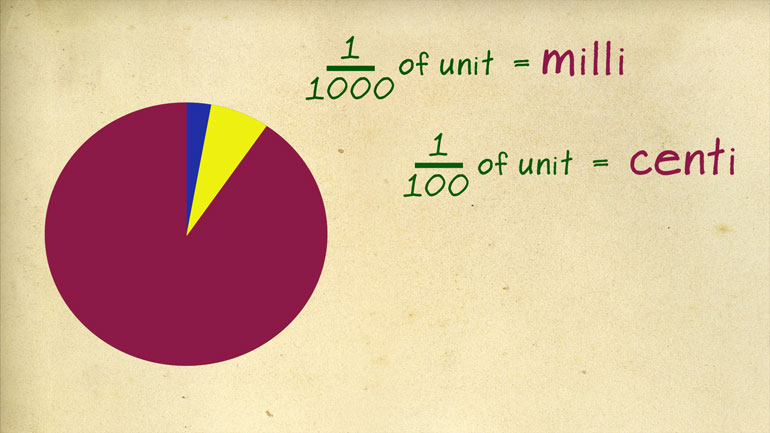ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Chemistry Videos 44 videos
Don't even think about stepping foot into a lab until you drink a nice big cup of safe-ty. Safety lesson number one: do not listen to this descript...
Today we're playing with fire. Wait, we're not supposed to say playing...having fun with fire? Today's lesson is on the colors that can be emitted...
These figures may not be significant to you, but they matter to us, okay? Oh, and to Science. They matter a ton to Science.
Chemistry: 2.5 Measuring in Metrics 189 Views
Share It!
Description:
Honestly, though. How seriously can you take the metric system when it measures things in moles? How heavy is a standard mole? How do you keep it from digging away?
Transcript
- 00:00
sweetie didn't we have some bad news for you people living in the good old US of
- 00:08
A well we're weird house elves only [USA holding scroll of parchment]
- 00:12
measure things with little measurements like pounds and yards and miles and
- 00:16
bunches well they're silly because they don't make a lot of sense there are 36 [Gorilla grabs a bunch of bananas]
- 00:21
inches in a yard 1,760 yards in a mile 5,280 feet in a mile and how's anyone
Full Transcript
- 00:29
supposed to remember all that nonsense but with the metric system there are 10
- 00:34
decimeters in a meter 10 centimeters in a decimeter and 10 millimeters in a
- 00:40
centimeter you feel in a sudden sense of calm walking over you yeah now that [Man standing on beach front]
- 00:45
would become associated with a rational orderly system of measurements and not
- 00:50
only does it make more sense but it's the system's measurement used all over
- 00:54
the world like we know it's cool to be different in all budgets sure would be [Gothic man walks by]
- 01:00
nice to be able to communicate with scientists in other countries than have
- 01:02
them understand what the heck we're talking about because this system of
- 01:05
unit they're so international it's actually called the International System [units of scientific measurements appear]
- 01:10
of Units 2x lever sublimating there you didn't see that one coming
- 01:15
well we call it SI for short and it's made up of a number of base unit that
- 01:19
can be used to measure all sorts of things including mass volume temperature
- 01:22
time and mold does not however measure athletic ability that's the other SI so [Man running on track]
- 01:27
let's go through the main base quantities real quick since you'll be
- 01:31
living eating and breathing these things for the rest of your chemistry career if [Woman sneezes while on the phone]
- 01:35
you're trying to measure the weight of something well you use kilograms created
- 01:39
by kg not to be confused with kilograms who are dangerous little old ladies like
- 01:45
to hang out in alleyways are to be avoided at all cost [Boy in alleyway and elderly woman holding a knife]
- 01:48
if measuring volume you'll use liter abbreviated by L yeah those soda
- 01:55
companies are already ahead of the curve if measuring time you'll use the second
- 02:00
abbreviated by s well okay at least we got something right for temperature [Man shrugs shoulders]
- 02:05
you'll be called upon to measure using kelvins abbreviated by K as a capital K
- 02:11
let's not insult this Kelvin fella making it lowercase and is measuring the [Kelvin appears]
- 02:15
amount of some substance you'll use the mole abbreviated mo L and it's not a
- 02:21
rodent the other thing you'll want to commit memory are the metric prefixes [Metric prefixes table appears]
- 02:26
these prefixes can be used with any SI unit although in practice we don't
- 02:30
really use them for temperature time or moles because we're not dealing with
- 02:33
extremely large or small numbers with those types of measurement unless you [Man running on track]
- 02:38
like to get your time measurements into a fraction of a nanosecond one tenth of
- 02:42
a regular unit is represented by the prefix deci so 1/10 of a meter is a
- 02:48
depth d meter one tenth of a liter is a deciliter and if you were normative one
- 02:54
hundredth of a unit is a send tip and one thousand is a milla going in the
- 03:00
other direction ten times a regular unit is deca as instead kamut ER or dekaliter
- 03:06
well 100 times is hecto and a thousand times is kilo like kilometer or Silla
- 03:13
leader so ya memorize those prefixes and you'll be good to go just don't ever [List of prefixes appear]
- 03:17
actually utter the words kill a pound so get laughed out of the lab and I'll [Scientists appear in a lab]
- 03:21
never be able to show your face again I'm assigning to the community
Related Videos
When you're about to marry the love of your life, not many things could stop you. However, finding out that your future hubby is keeping his crazy...
Here at Shmoop, we work for kids, not just the bottom line. Founded by David Siminoff and his wife Ellen Siminoff, Shmoop was originally conceived...
ACT Math: Elementary Algebra Drill 4, Problem 5. What is the solution to the problem shown?
AP® English Literature and Composition Passage Drill 1, Problem 1. Which literary device is used in lines 31 to 37?
AP® English Literature and Composition Passage Drill 2, Problem 1. What claim does Bacon make that contradicts the maxim "Whatsoever is delig...