ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Science Videos 686 videos
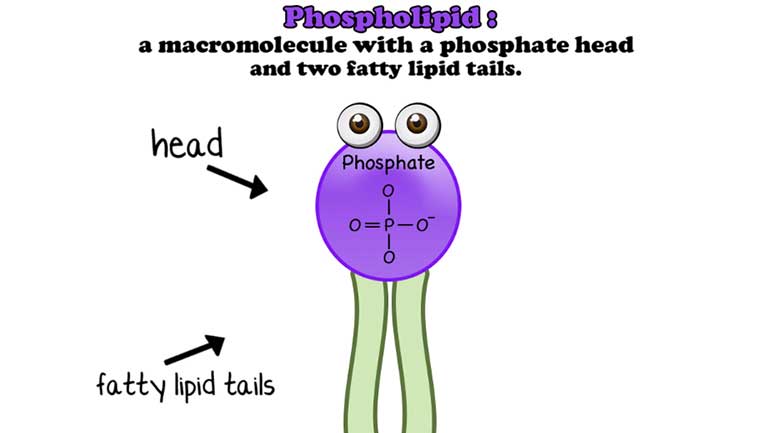
Anything that has a cell (bacteria, listen up!) has phospholipids that keep the cell contained and give it form and shape. Phospholipids protect us...
GMOs. Now that’s a scary word. Or is it? Guess it’s time to ask ourselves: WWMST? ...For those of us who don’t constantly ask ourselves “wh...
AP Biology 1.1 Free Energy and Molecular Building Blocks 1115 Views
Share It!
Description:
AP Biology: Free Energy and Molecular Building Blocks Drill 1, Problem 1. Which statement incorrectly describes the properties of water?
Transcript
- 00:03
Here's your shmoop du jour...a la Bio.
- 00:07
Which statement incorrectly describes the properties of water?
- 00:13
And here are the potential answers...
- 00:18
Water. H20. That stuff we're all supposed to drink 8 glasses of every day.
- 00:23
Even those of us with a drinking problem.
Full Transcript
- 00:27
But aside from hydrating our body, it has some other really cool, unique properties
- 00:30
that are good to know for the AP bio test. And since this is a "which statement incorrectly
- 00:36
describes" question...
- 00:37
...we can just look at every single answer choice to determine which one ain't doin'
- 00:42
it for us. Starting with our first answer choice...is
- 00:45
water a nonpolar molecule? Well, what does that mean? Does it just like
- 00:50
the thermostat cranked up to 90? Nope... polarity in science refers to differences
- 00:55
in charge or ELECTRONEGATIVITY between atoms in a molecule.
- 01:02
Electronegativity means the tendency to attract electrons. With or without makeup.
- 01:08
In this problem, it means that if our water molecule were to have a different electronegativity
- 01:13
around oxygen compared to its two hydrogen atoms, the molecule would be polar.
- 01:18
Hm ok...so we know that Oxygen is a super electronegative atom, and attracts lots of
- 01:25
negative charge to it. It's something we should just know and memorize
- 01:30
because it's one of those things that is just uber-important.
- 01:33
Hydrogen, on the other hand, does not have a high electronegativity. Sorry, hydrogen.
- 01:38
We're sure you make up for it by having a great personality.
- 01:43
So even though there are two hydrogen atoms for one oxygen in every water molecule...
- 01:48
...the water molecule is still super negative around the oxygen and is unbalanced in charge.
- 01:54
This difference in charge makes water POLAR. So our answer is A.
- 01:58
Now start chugging. You've still got 6 glasses to go today.
Related Videos
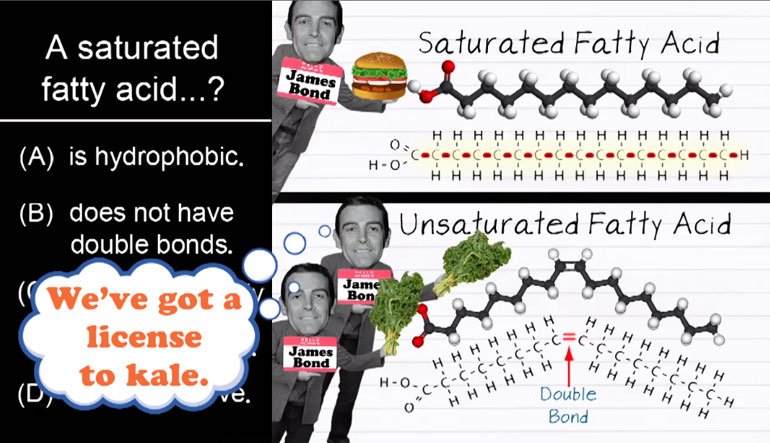
AP Biology: Biological System Interactions Drill 1, Problem 1. Complete the sentence about a saturated fatty acid.
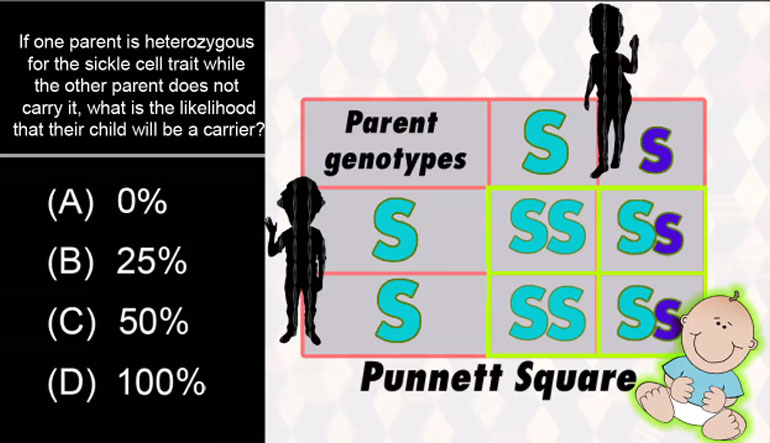
AP Biology: Essential Life Process Information Drill 1, Problem 1. If one parent is heterozygous for the sickle cell trait while the other par...
AP Biology: Evolution Drives the Diversity and Unity of Life Drill 1, Problem 1. The first cells on planet Earth were likely what?
AP® Biology: Evolution Drives the Diversity and Unity of Life Drill 1, Problem 2. What was likely the first genetic material?
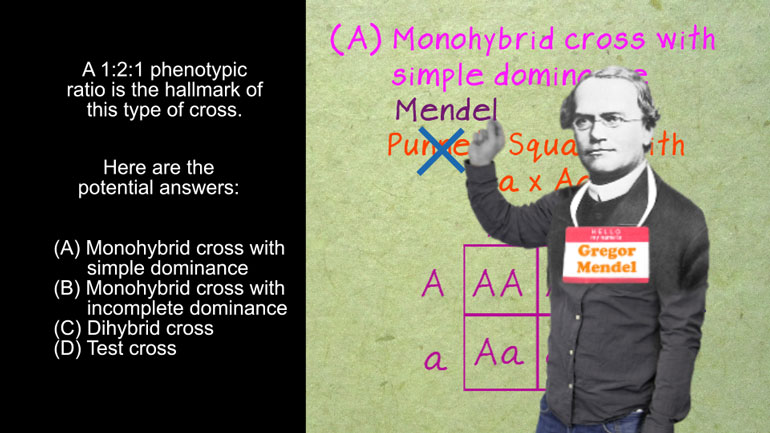
AP Biology: Evolution Drives the Diversity and Unity of Life Drill 1, Problem 4. Hardy-Weinberg equilibrium requires that a population meet al...