ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Test Prep Videos 443 videos
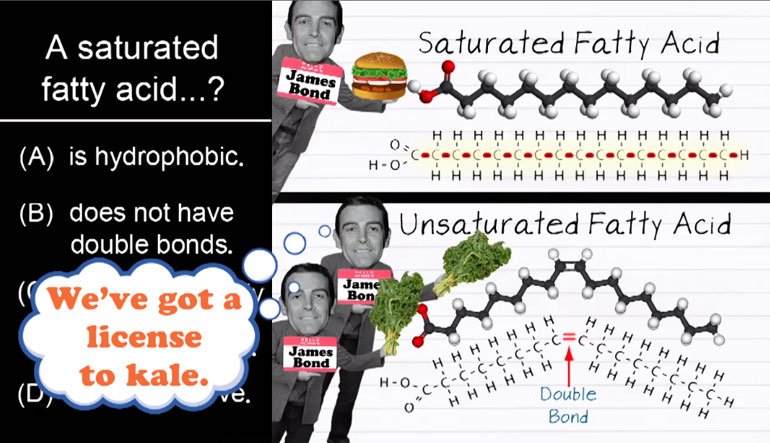
AP Biology: Biological System Interactions Drill 1, Problem 1. Complete the sentence about a saturated fatty acid.
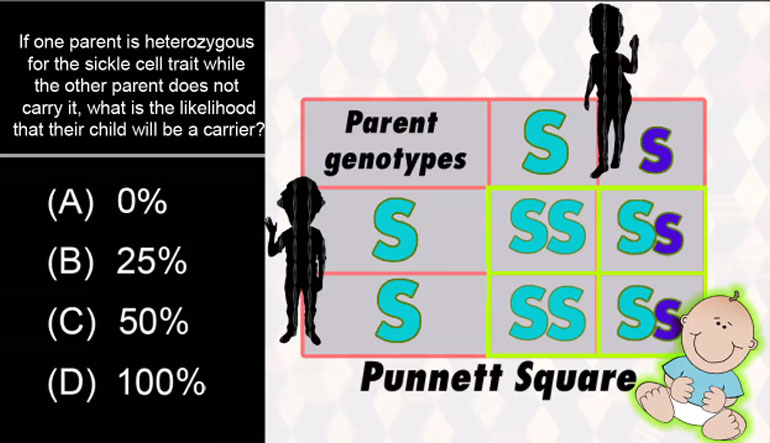
AP Biology: Essential Life Process Information Drill 1, Problem 1. If one parent is heterozygous for the sickle cell trait while the other par...
AP Biology: Evolution Drives the Diversity and Unity of Life Drill 1, Problem 1. The first cells on planet Earth were likely what?
AP Chemistry 1.1 Forming and Breaking Bonds 29 Views
Share It!
Description:
AP Chemistry 1.1 Forming and Breaking Bonds. Find the conjugate base.
Transcript
- 00:04
And here's your Shmoop du jour, brought to you by baking soda.
- 00:08
Its use in your bread is… on the rise. Okay, here's today’s question. [Bread expands out of microwave]
- 00:12
In our bodies, we have a buffering system to keep our pH at a certain level. This buffering
- 00:16
system uses carbonate and bicarbonate ions. What is the conjugate base of the bicarbonate
- 00:22
ion, HCO3- in water?
Full Transcript
- 00:27
And here are your potential answers.
- 00:32
Sodium bicarbonate is the active ingredient in baking soda, and it’s what causes bread [People scared of bread expanding from a microwave]
- 00:37
to rise. Or to take over your oven and become some
- 00:40
sort of crazy dough monster. Hypothetically… For this question, we need to consider the [Acid and Base appear at a table]
- 00:45
acid-base chemistry of the bicarbonate ion.
- 00:48
Specifically, we want to know the conjugate base of this species.
- 00:51
Acids and bases react to form their conjugate partners. [Acid and base getting married]
- 00:54
When an acidic species H A reacts with a base B by donating a proton, it becomes its conjugate
- 01:00
base, A-.
- 01:01
On the other hand, when a basic species B reacts with an acid H A by accepting a proton, [Base accepting proton]
- 01:07
it becomes its conjugate acid, HB+.
- 01:09
And then it goes to Starbucks for a pumpkin spice latte. [Base drinking a starbucks latte]
- 01:13
Get it? Basic? Heh.
- 01:15
Anyway, we need to know what happens when bicarbonate acts as an acid, and thus becomes [Bicarbonate jumping on a sofa]
- 01:20
its conjugate base. To act as an acid, it must lose a proton.
- 01:24
Don’t forget that when we split up this ionic species, we have to consider the resulting [Ionic species split in half]
- 01:28
charges.
- 01:30
Our entire bicarbonate species has a -1 charge. Our proton, H+, has a +1 charge.
- 01:37
That means that the CO3 species must have a -2 charge, as the sum of the charge on this
- 01:43
and the proton must equal the overall charge on the entire bicarbonate ion.
- 01:47
So we can see that our conjugate base is CO32-.
- 01:51
So C is the correct answer. And if you understood all that, then you definitely [Girl eating a cake]
- 01:55
deserve some kind of baked good. We recommend cookies, but whatever floats
- 01:59
your boat.
- 02:00
- 02:01
- 02:02
Related Videos
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?
AP Chemistry 3.2 Laws of Thermodynamics. What is the value for ΔG?