ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Test Prep Videos 443 videos
ACT Science: Research Summary Passage Drill 2, Problem 1. Why do you think that the filter paper will not remove the salt from the water?
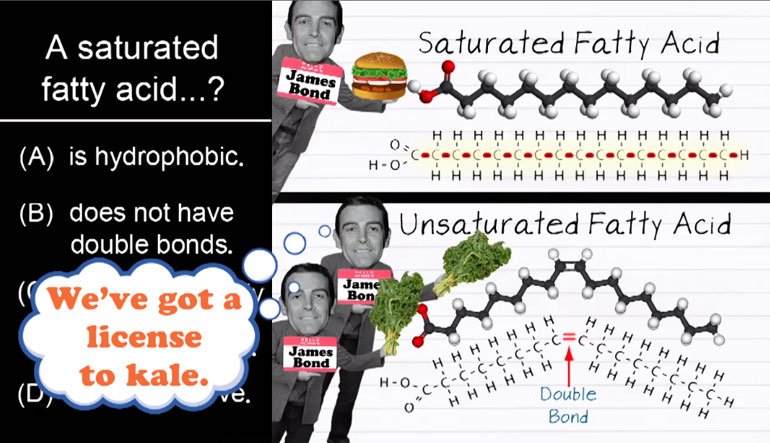
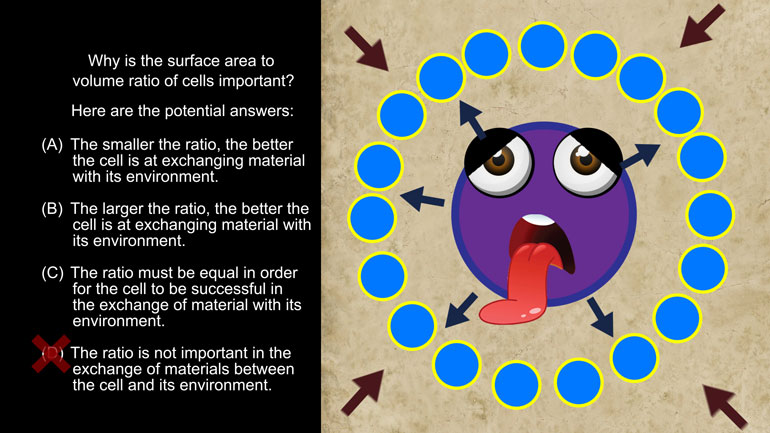
AP Biology: Biological System Interactions Drill 1, Problem 1. Complete the sentence about a saturated fatty acid.
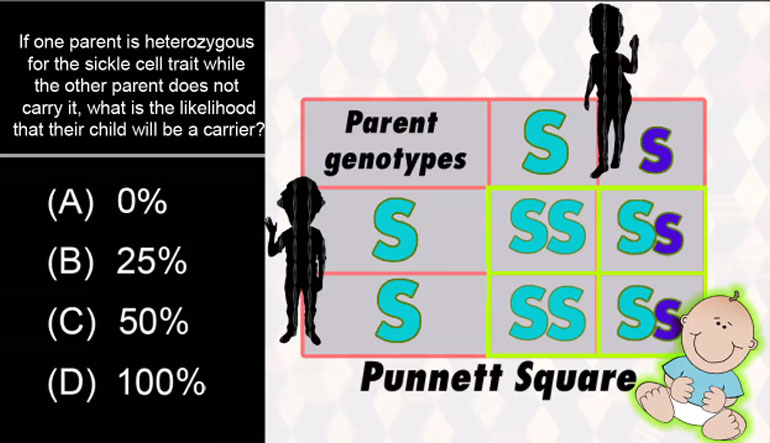
AP Biology: Essential Life Process Information Drill 1, Problem 1. If one parent is heterozygous for the sickle cell trait while the other par...
AP Chemistry 3.2 Chemical Reaction Rates 6 Views
Share It!
Description:
AP Chemistry 3.2 Chemical Reaction Rates. What is the experimental rate law for the reaction?
Transcript
- 00:04
Here’s your Shmoop du jour, brought to you by experimental rate law, the rule that made
- 00:09
all speed limits Avogadro’s number for a day. [Policeman pointing to speed limit with Avogadro's number on it]
- 00:12
It wasn’t popular.
- 00:13
Anyway, here’s today’s question:
- 00:15
What is the experimental rate law for the following reaction, given the data below?
Full Transcript
- 00:20
And here are your potential answers: Anybody feeling psychic? [Psychic woman appears]
- 00:27
Let’s start off by trying to predict what that rate law should be based on the reaction [Stoichiometry appears on her crystal ball]
- 00:31
stoichiometry.
- 00:33
So the stoichiometric coefficients are often the same as the reaction
- 00:37
order for each reactant. [Man working in a science lab]
- 00:39
Since the reactants are two CO and one O2, the reaction will probably be second order [Finger points to the reactants in the equation]
- 00:44
in CO and first order in O2.
- 00:47
If this guess is correct, the rate equals the
- 00:50
rate constant times the concentration of carbon monoxide squared times the concentration of [Psychic woman holding the rate constant]
- 00:56
oxygen, which is answer (B).
- 00:58
Shh…something is telling us we should open a psychic hotline…call now…only $10 a [Psychic woman doing a phone line advert]
- 01:03
minute…
- 01:04
But unfortunately, this isn’t a guessing game.
- 01:06
To test our hypothesis, we’ll need to look at the table. [Wheel of fortune presenter chucks away his microphone and walks off]
- 01:09
Between experiments 1 and 2, the initial concentration of CO is doubled while the initial concentration
- 01:15
of O2 stays the same. [Man pointing to figures in a table]
- 01:17
These two data points show us how the rate depends on the concentration of CO without
- 01:22
the influence of O2.
- 01:23
Seriously, no peer pressure here.
- 01:25
In this case, when the CO concentration is doubled, the rate increases by a factor of
- 01:30
four, which means the rate varies with concentration of CO squared. [Person using a pipette in a lab]
- 01:36
Between experiments 1 and 3, the initial concentration of O2 is doubled while the concentration of
- 01:40
CO stays the same.
- 01:42
We can use these two data points to figure out how the rate depends on the concentration
- 01:45
of O2 without worrying about CO.
- 01:47
Here, we see that when the O2 concentration is doubled, the reaction rate is also doubled,
- 01:52
so the rate is proportional to the O2 concentration. [Person taking blue liquid out of a flask]
- 01:55
Putting this all together, our overall rate law is rate = k[CO]2 [O2] the rate equals [Man pointing to a blackboard with the answers on]
- 01:59
the rate constant times the concentration of carbon monoxide squared times the concentration
- 02:04
of oxygen.
- 02:05
And, hey…that sounds pretty familiar, which means our guess was right and B is the correct [Psychic woman looks happy]
- 02:10
answer.
- 02:11
All right, well, this was fun, but we gotta get this hotline set up…shh…the spirits
- 02:16
are speaking…they're telling you to phone in and stay on the line for an hour…. [Psychic hotline ad]
- 02:21
on your parents credit card... yes, 1-800 shmoop, we'll tell you your future...
Related Videos
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?
AP Chemistry 3.2 Laws of Thermodynamics. What is the value for ΔG?