ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Test Prep Videos 443 videos
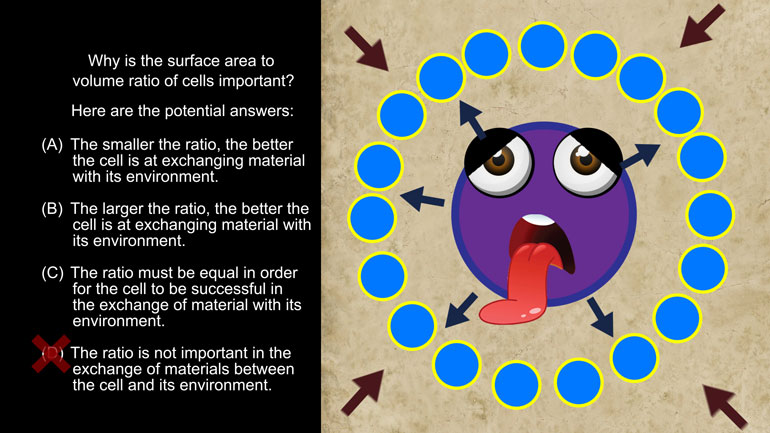
ACT Science: Research Summary Passage Drill 2, Problem 1. Why do you think that the filter paper will not remove the salt from the water?
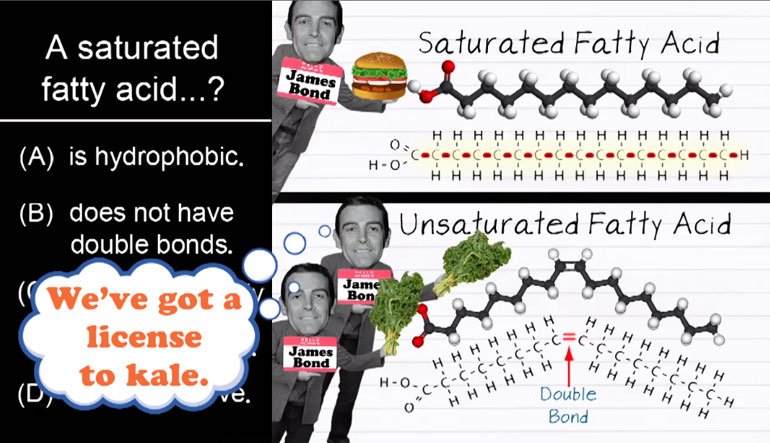
AP Biology: Biological System Interactions Drill 1, Problem 1. Complete the sentence about a saturated fatty acid.
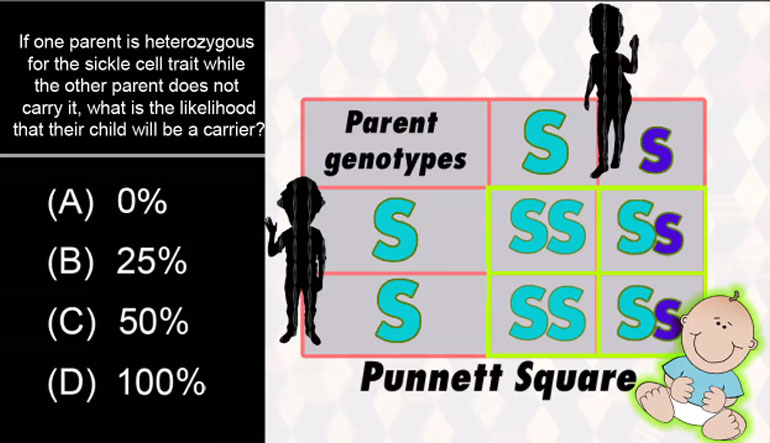
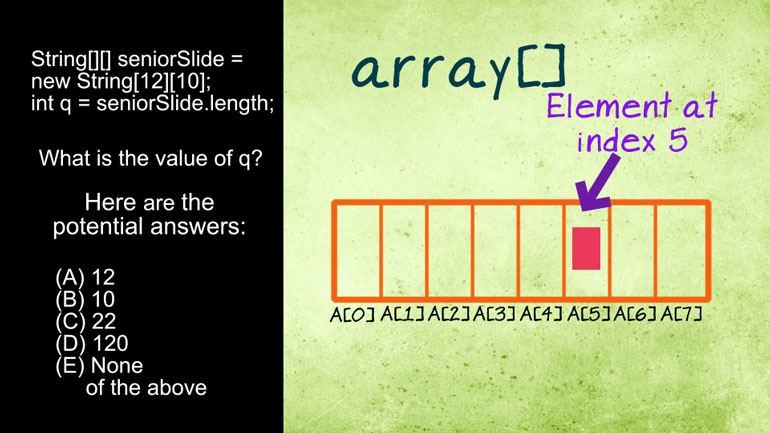
AP Biology: Essential Life Process Information Drill 1, Problem 1. If one parent is heterozygous for the sickle cell trait while the other par...
AP Chemistry 2.1 Chemical Reaction Rates 10 Views
Share It!
Description:
AP Chemistry 2.1 Chemical Reaction Rates. Which of the following is true according to the reaction profile?
Transcript
- 00:04
And here’s your Shmoop du jour, brought to you by humps, also known as “lovely science [Camel appears in a desert]
- 00:09
lumps.”
- 00:10
Okay, here’s our question:
- 00:11
Which of the following is true according to the following reaction profile?
- 00:14
Full Transcript
- 00:15
And here are your potential answers: Okay, so though it may just look like a random [Boy drawing]
- 00:22
squiggle a kindergartener drew, what you’re looking at is actually our reaction profile
- 00:27
curve, the focus of today’s problem.
- 00:30
Figure out what the deal with this curve is and we figure out the problem.
- 00:33
If only that was the way all problems in life worked… [Woman discussing problems with a reaction curve]
- 00:36
Believe it or not, a reaction profile isn’t the side view of a reaction.
- 00:40
It’s a graphical representation of the energy pathway through a chemical reaction. [Example of a reaction profile graph]
- 00:45
The horizontal axis shows the reaction progress, which means how close the reaction is to completion.
- 00:53
Just like in a chemical reaction equation, the left side of this graph represents the
- 00:58
reactants, and the right side represents the products. [Graph showing reactants and products of a reaction]
- 01:00
So as long as you’re used to reading left-to-right, it should be pretty easy.
- 01:05
Sorry, manga enthusiasts and Hebrew-speakers.
- 01:07
Anyway, the vertical axis is the energy of the reactant and product species.
- 01:13
You can think of it as the species in this reaction riding that curve like a roller coaster [People riding a roller-coaster]
- 01:18
as they’re transformed from the reactants into the products.
- 01:21
Woo!
- 01:22
Hope you didn’t eat a big lunch.
- 01:25
Since there are two humps here, we know that the reaction occurs in two steps. [Two humps of reaction curve highlighted]
- 01:29
And that maybe the kindergartner who drew this doodle is a camel-lover.
- 01:32
Anyway, the three local minima represent the reactants, intermediates, and products.
- 01:38
Each local maximum represents a transition state.
- 01:42
So let’s dive back into those answers. [People dive into a river]
- 01:45
Is this an endothermic reaction?
- 01:47
An endothermic reaction is a reaction that absorbs energy as heat from the surroundings.
- 01:52
From the reaction profile curve, we can see that the energy of the products is lower than
- 01:56
the energy of the reactants.
- 01:59
That means that the reaction releases energy into the surroundings in the form of heat. [Exothermic reaction definition]
- 02:04
Which means two things: It’s great to take on ski trips, AND it’s
- 02:07
exothermic, not endothermic.
- 02:08
So we can give option B the cold shoulder. [Letter B zapped and frozen]
- 02:12
Is the first step of the reaction the rate-determining step?
- 02:15
Well, out of this reaction’s two steps, the rate-determining step will be the one
- 02:19
that has the highest activation energy barrier to overcome.
- 02:24
The barrier to the first step is the difference in energy between the reactants and the first
- 02:28
transition state.
- 02:30
The barrier to the second step is the difference in energy between the intermediates and the
- 02:35
second transition state.
- 02:37
In this case, the second barrier is larger, so the second step is rate-determining. [Finger points to second transition state]
- 02:40
Looks like we’ve just determined that option C is not our guy.
- 02:45
How about D?
- 02:46
Was a catalyst used to decrease the activation energy of the reaction? [Catalyst in court with a judge]
- 02:50
Well, we have no way to tell whether a catalyst was already involved in this reaction or not.
- 02:55
Not enough information, not our answer.
- 02:57
So that means that A - “There is an intermediate formed in the reaction,” is the correct
- 03:04
answer.
- 03:05
We figured that out like a minute ago, but it’s good to consider the other options
- 03:07
just to be sure.
- 03:08
Well…that and we didn't have any other cool plans for today, and we figured we'd force [Two guys sitting on a sofa]
- 03:11
you to spend more time with us.
- 03:12
That's what friends do, right?
- 03:13
…Right?
Related Videos
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?
AP Chemistry 3.2 Laws of Thermodynamics. What is the value for ΔG?