ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Test Prep Videos 443 videos
ACT Science: Research Summary Passage Drill 2, Problem 1. Why do you think that the filter paper will not remove the salt from the water?
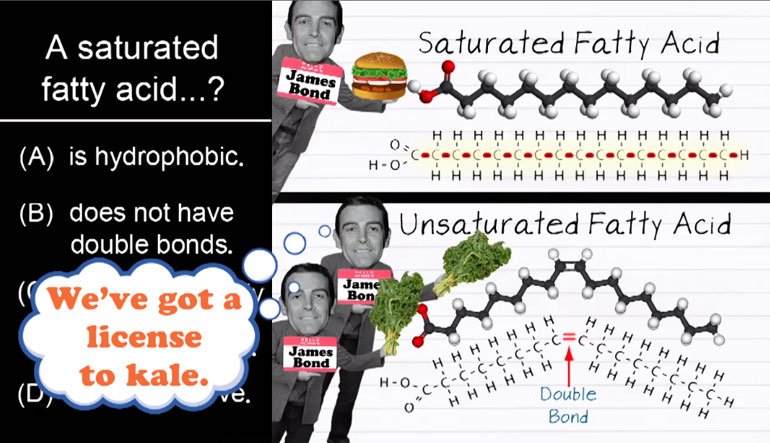
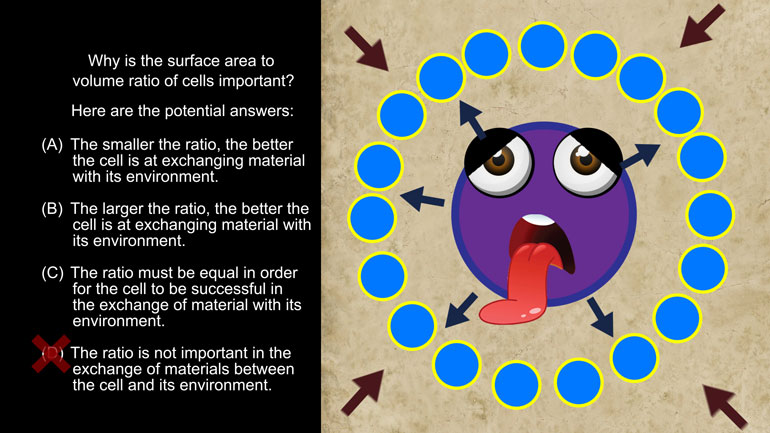
AP Biology: Biological System Interactions Drill 1, Problem 1. Complete the sentence about a saturated fatty acid.
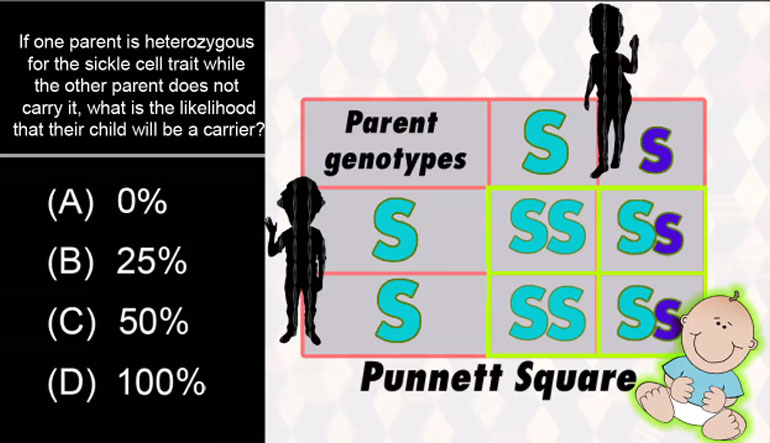
AP Biology: Essential Life Process Information Drill 1, Problem 1. If one parent is heterozygous for the sickle cell trait while the other par...
AP Chemistry 3.3 Chemical Reaction Rates 3 Views
Share It!
Description:
AP Chemistry 3.3 Chemical Reaction Rates. Which of the following is true regarding rates of chemical reactions?
Transcript
- 00:04
And here’s your Shmoop du jour, brought to you by decreasing the temperature.
- 00:07
Turn down for what? …because it’s too hot in here. [Man crushes a table and dances]
- 00:11
Here’s today’s question:
- 00:13
Which of the following is true regarding rates of chemical reactions?
- 00:16
And here are your potential answers:
Full Transcript
- 00:23
This question is about reaction rates, by which we don’t mean how long it takes you [Man on stage telling jokes]
- 00:28
to start laughing after we tell a hilarious joke.
- 00:30
We’re talking about chemistry here, guys.
- 00:33
Get with it.
- 00:34
There are a few tried and true ways to speed up chemical reaction rates. [Scientist transferring substance to a beaker and it explodes]
- 00:38
Let’s imagine that each time two of these red molecules react, they create one blue [Red molecules collide and create blue molecule]
- 00:43
molecule.
- 00:44
To increase the reaction rate, we could increase the concentration of the reactants.
- 00:48
This would lead to more collisions between the reacting molecules, which would cause [Molecules colliding]
- 00:53
the reaction rate to increase.
- 00:55
That’s pretty straightforward, right?
- 00:57
Another way we could increase the reaction rate would be to use a catalyst.
- 01:01
Because that’s the whole point of catalysts.
- 01:02
Seriously.
- 01:03
That’s all they do.
- 01:04
They’re kind of a one-trick pony.
- 01:07
Catalysts work by decreasing activation energy barriers so molecules that collide are more [Catalysts in a beaker with molecules]
- 01:13
likely to react.
- 01:14
And if you’ve ever witnessed two angry people bumping into each other, you’ll know that [Two guys bump into each other]
- 01:18
people that collide are also pretty likely to react.
- 01:22
And last, but not least, we could also speed up a reaction by turning up the heat. [Fire burning]
- 01:28
Besides keeping us nice and toasty, increasing the temperature can speed up a chemical reaction
- 01:34
by giving reacting molecules more kinetic energy.
- 01:37
This makes them collide more frequently and with more energy, so they’re more likely [Molecules colliding frequently]
- 01:41
to overcome the activation barrier and react.
- 01:44
Which is why if you go to a hot yoga class, you’ll see a lot of people falling over [Boy in yoga class falls over]
- 01:48
each other.
- 01:49
That’s how that works, right?
- 01:50
Hey, that last method sounds really similar to answer B, which says that chemical reaction
- 01:55
rates can be increased by increasing the temperature.
- 02:00
And wouldn't you know it, the answer is B. Did you get it right? [Man with hair on fire running and screaming]
- 02:03
We bet you did.
- 02:04
You are on fire today. [Man uses fire extinguisher on mans head]
Related Videos
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?
AP Chemistry 3.2 Laws of Thermodynamics. What is the value for ΔG?