ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Test Prep Videos 443 videos
ACT Science: Research Summary Passage Drill 2, Problem 1. Why do you think that the filter paper will not remove the salt from the water?
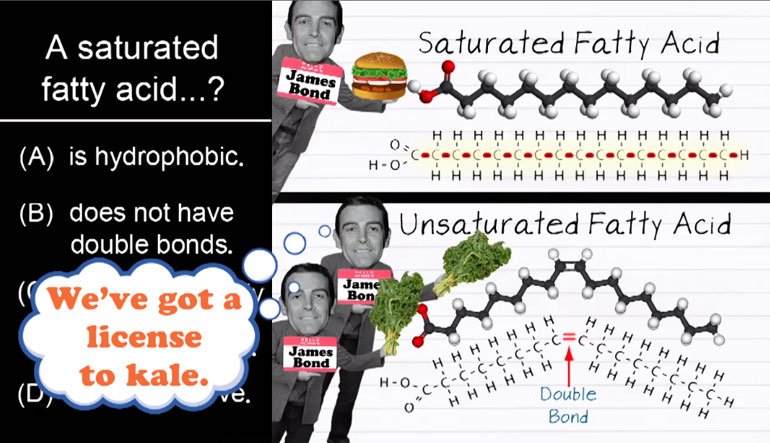
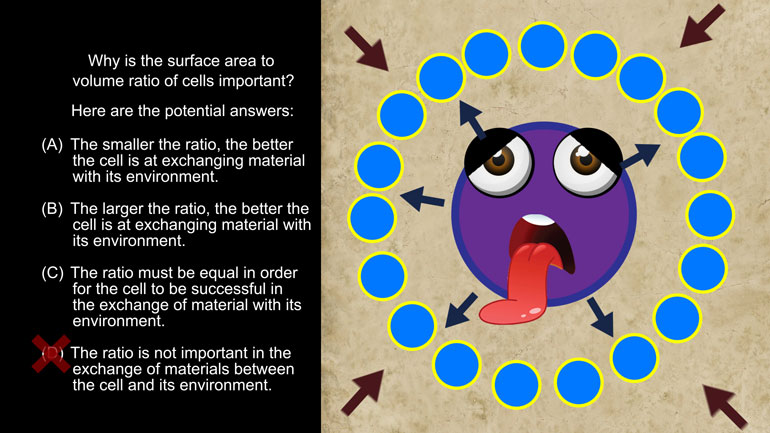
AP Biology: Biological System Interactions Drill 1, Problem 1. Complete the sentence about a saturated fatty acid.
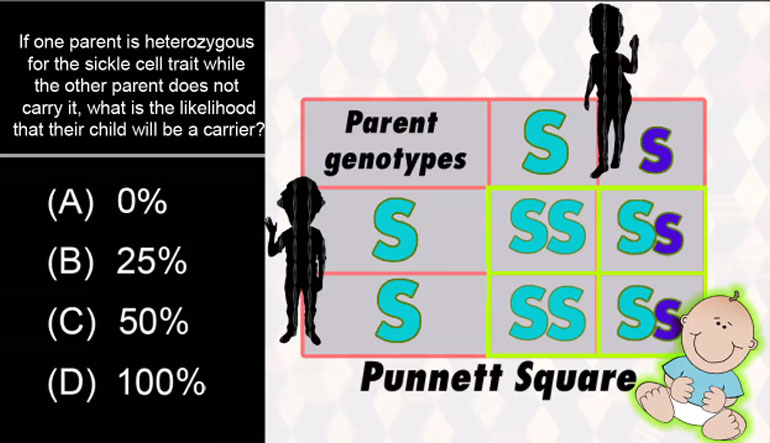
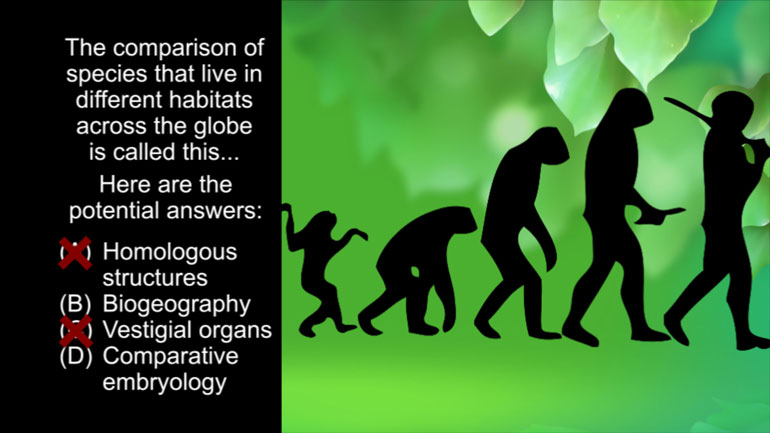
AP Biology: Essential Life Process Information Drill 1, Problem 1. If one parent is heterozygous for the sickle cell trait while the other par...
AP Chemistry 2.2 Chemical Reaction Rates 26 Views
Share It!
Description:
AP Chemistry 2.2 Chemical Reaction Rates. What would happen to the reaction?
Transcript
- 00:03
Here’s your Shmoop du jour, brought to you by rockets.
- 00:06
They rock so much, they put it in the name. [Engineers working on a rocket]
- 00:10
Okay, here’s our question:
- 00:12
Nitrogen dioxide is a substance that is sometimes used in rocket fuel.
- 00:16
Nitrogen dioxide is prepared according to the following reaction, riiiiight…here….
Full Transcript
- 00:22
And the rate law for this reaction is Rate = k[NO]2[O2].
- 00:26
< The rate constant times the concentration of NO times the concentration of o2> [Rate law and reaction example]
- 00:32
What would happen to the rate of the reaction if the concentration of NO were doubled, and
- 00:37
the concentration of O2 remained the same?
- 00:40
And here are the potential answers: [mumbling]
- 00:42
Before we launch right into this problem, let’s lift off our spirits with this fuel for [Boy tied to a rocket and the rocket sets off]
- 00:48
thought.
- 00:49
Many NASA astronauts have been chemists, so if you stick with this chemistry stuff, and
- 00:54
take some time to planet all out, you too could become an astronaut…[woman astronaut holding chemical substance and it explodes]
- 00:58
And that would be out of this world.
- 00:59
Now that we’ve run out of space for these astronomically terrible puns, let’s get [woman lab worker holding two erlenmeyer flasks]
- 01:04
back to the problem.
- 01:06
We’re given the rate law for the rocket fuel synthesis reaction.
- 01:11
The rate equals the concentration of NO squared times the concentration of O2.
- 01:15
The concentration of O2 is kept constant.
- 01:18
If we double the concentration of NO into this equation, the rate will increase by a
- 01:21
factor of four, since the concentration of NO is squared in the rate law.
- 01:26
And what that means is we don't have to drag anything out and we can just go ahead and [Students yawning in a classroom]
- 01:31
tell you that the correct answer is (D), “The rate would quadruple.”
- 01:34
Phew.
- 01:35
Isn't it nice when things are simple?
- 01:36
Like going into space.
- 01:37
It's easy-peasy, right?
- 01:38
But uh…just to be on the safe side, maybe you should trade in that rocket and get your [Man lands in a store on a rocket and demands a refund for his car]
- 01:42
car back.
Related Videos
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?
AP Chemistry 3.2 Laws of Thermodynamics. What is the value for ΔG?