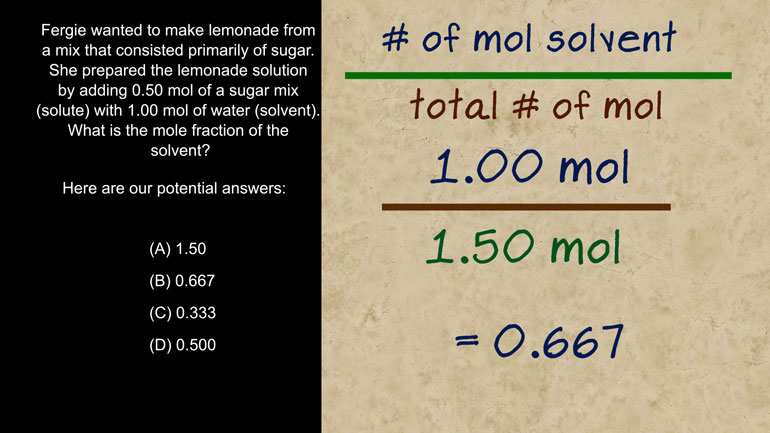ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Test Prep Videos 443 videos
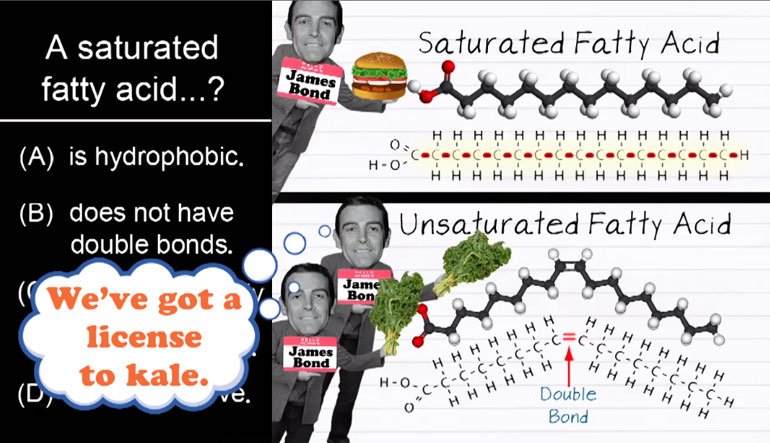
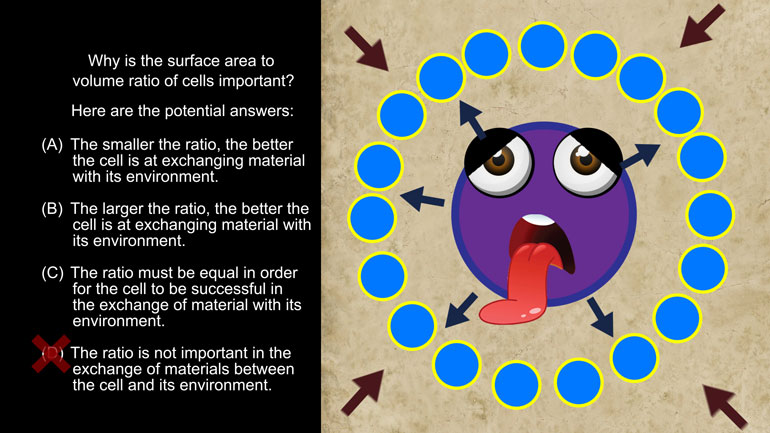
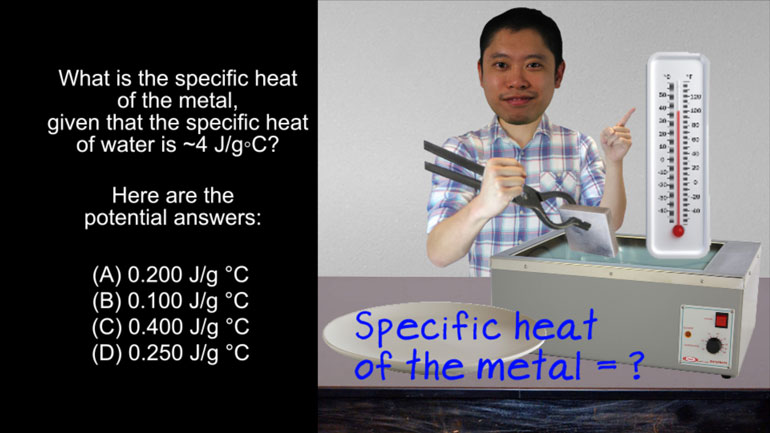
AP Biology: Biological System Interactions Drill 1, Problem 1. Complete the sentence about a saturated fatty acid.
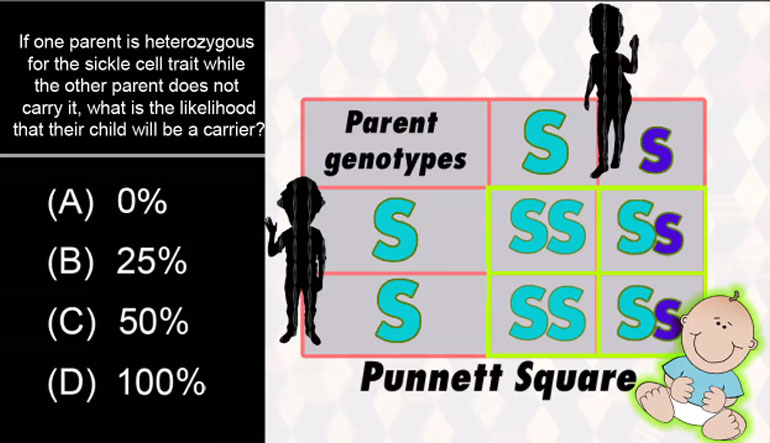
AP Biology: Essential Life Process Information Drill 1, Problem 1. If one parent is heterozygous for the sickle cell trait while the other par...
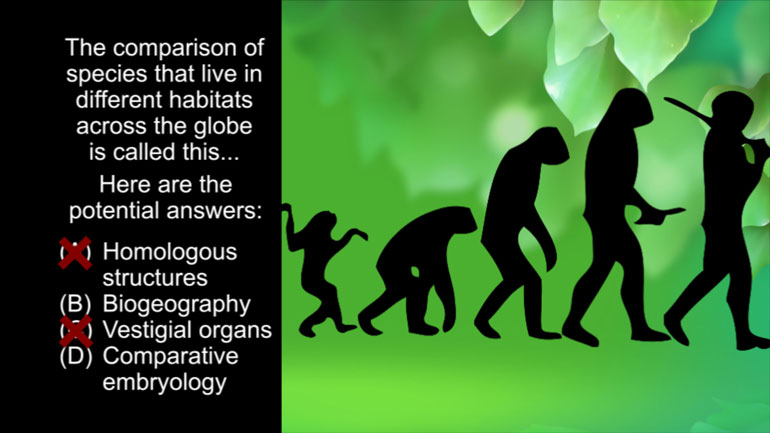
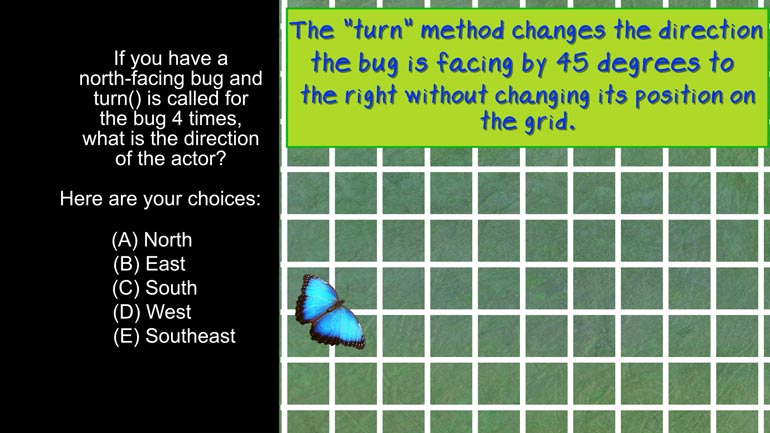
AP Biology: Evolution Drives the Diversity and Unity of Life Drill 1, Problem 1. The first cells on planet Earth were likely what?
AP Chemistry 1.5 Forming and Breaking Bonds 8 Views
Share It!
Description:
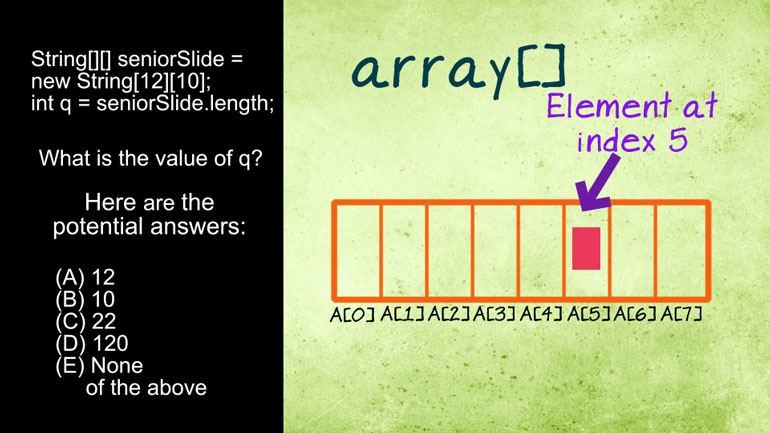
AP Chemistry 1.5 Forming and Breaking Bonds. What can we expect to see with regard to the current stage of this reaction?
Transcript
- 00:03
Here’s your Shmoop du jour, brought to you by the scientific Time Warp. [Clock ticks rapidly forward]
- 00:08
It's just a shift to the left, and then a shift to the right.
- 00:11
Okay, enough Time Warping…here’s our question:
- 00:14
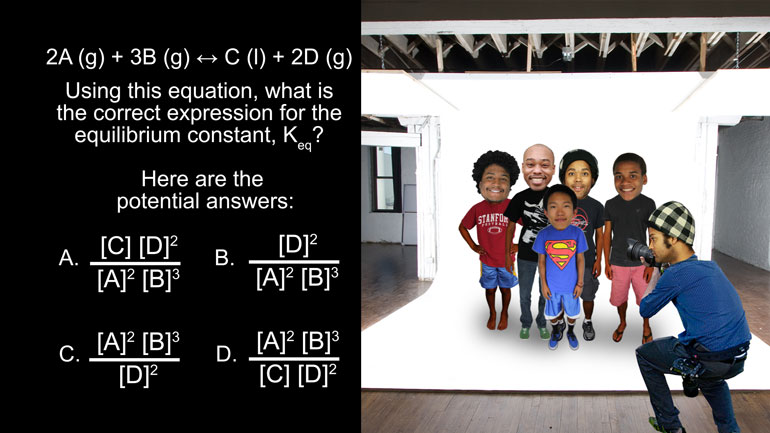
Consider the following chemical reaction equation…
- 00:20
Using this equation, if we removed the entire D product from the reaction, what can we expect
Full Transcript
- 00:25
to see with regard to the current stage of this reaction?
- 00:28
And here are the potential answers:
- 00:31
To solve this problem, we need to remember Le Châtelier's Principal. [Person photographing men on stage]
- 00:36
Thank you, 1860s French high school principal Monsieur Talbert.
- 00:42
We know Henri Louis Le Châtelier couldn’t have [Henri working in a lab]
- 00:44
developed his chemical equilibrium law without the nurturing school environment you helped
- 00:49
to create for him.
- 00:50
Bravo.
- 00:51
Now that that’s out of the way, we also need to remember Le Châtelier's Principle
- 00:54
of chemical equilibrium, it says that when a chemical system in dynamic equilibrium is [Teacher writing on the board]
- 01:02
perturbed by changing the conditions, the equilibrium changes to counteract the perturbation.
- 01:11
In our chemical reaction, if we remove all of the product D, then Le Châtelier's Principle [Product D removed from chemical reaction]
- 01:16
tells us that the reaction will adjust to counteract that change by creating more of
- 01:20
the product D.
- 01:22
This would correspond to the reaction proceeding from left to right. [Blue ball moves from left to the right]
- 01:24
It’s kind of like a clown car packed with clowns.
- 01:27
…Which…come to think of it, really can’t be safe. [Clowns enters small car and drives away]
- 01:30
But anyway.
- 01:31
If all the clowns in the back seat get out, some of the other clowns will move to the
- 01:34
back so the driver can see the road.
- 01:36
…Seriously, how have police not cracked down on this yet?
- 01:39
Here’s another way to think about it.
- 01:40
If our chemical reaction is at dynamic equilibrium, the rate of the forward reaction is exactly
- 01:45
the same as the rate of the reverse reaction. [D reactants in the woods covered in barbed wire]
- 01:48
If all of species D were removed, the forward reaction could still proceed at the same rate,
- 01:54
but the backward reaction could not take place at all since there would be no D available
- 01:58
to react.
- 01:59
As a result, the overall net reaction would proceed from left to right.
- 02:02
So, we can see that the reaction will move from left to right.
- 02:06
Another way to say the same thing is that the equilibrium will shift to the right.
- 02:10
This just happens to be answer choice (A), which is the right answer. [Answer A circled green]
- 02:14
And now if you'd excuse us, we have a few citizens' arrests to make. [Police arresting man riding scooter]
- 02:16
Keeping the roads safe, one clown car at a time.
Related Videos
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?
AP Chemistry 3.2 Laws of Thermodynamics. What is the value for ΔG?