ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Test Prep Videos 443 videos
ACT Science: Research Summary Passage Drill 2, Problem 1. Why do you think that the filter paper will not remove the salt from the water?
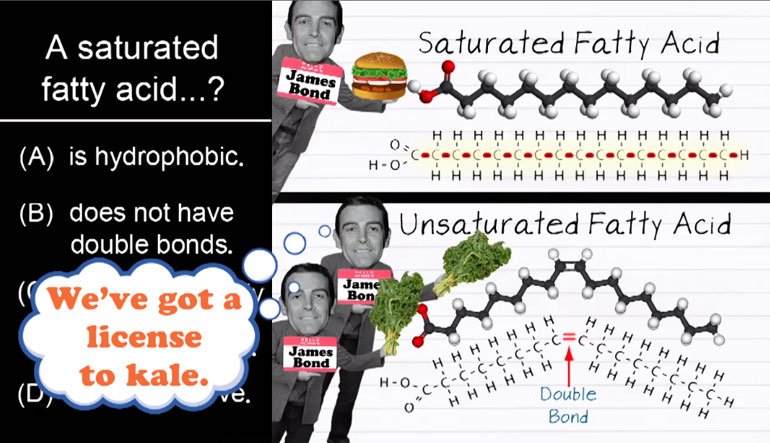
AP Biology: Biological System Interactions Drill 1, Problem 1. Complete the sentence about a saturated fatty acid.
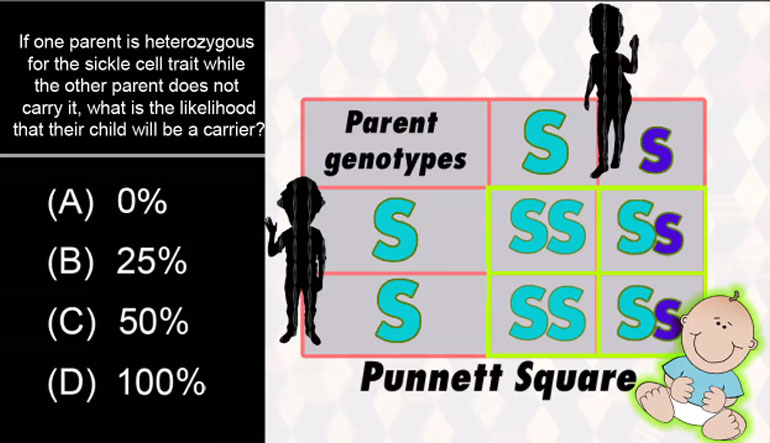
AP Biology: Essential Life Process Information Drill 1, Problem 1. If one parent is heterozygous for the sickle cell trait while the other par...
AP Chemistry 2.4 Chemical Reaction Rates 8 Views
Share It!
Description:
Time to calculate some half-life. And no, you can't only half-pay attention.
Transcript
- 00:04
And here’s your Shmoop du jour, brought to you by carbon dating, the Match.com for
- 00:09
scientists. We guarantee we’ll find you someone you’ll [Scientist using a tablet for match.com]
- 00:11
have chemistry with. Okay, here’s today’s question:
- 00:14
Half-life is a measure of the time it takes for half a sample to decay. Radiometric carbon
- 00:19
dating uses the half-life of carbon-14 to estimate the age of a carbon-containing objects.
Full Transcript
- 00:24
What is the rate constant for a first-order reaction that has a half-life of 56.0 seconds?
- 00:30
And here are your potential answers:
- 00:33
So what is “half-life,” aside from the portion of your time you spend on Netflix? [Woman with feet up watching TV]
- 00:39
For the sake of this problem, “half-life” is the amount of time it takes for something
- 00:42
to decrease to half its initial value. As the problem tells us, in chemistry, “half-life”
- 00:47
often means how long it takes for half of a radioactive sample to decay, but it can [Sample decays and clock ticks]
- 00:52
also refer to how long it takes for half of some species to be consumed in a regular chemical
- 00:56
reaction. In this problem, we know that some species [Woman zaps magic stick to a man on stage and he disappears]
- 00:59
is disappearing following a first-order rate law, and half of it is gone after 56.0 seconds.
- 01:05
To find the rate constant, we have to remember the equation for half-life: [Girl using calculator]
- 01:09
t½ = 0.693/k, where k is the first-order rate constant.
- 01:18
If you don’t remember this equation, you can figure it out by starting with the rate
- 01:21
law and solving a first order ordinary differential equation.
- 01:25
That is, if you want to make life way harder for yourself. [Boy studying at his desk]
- 01:27
Might just want to take the plunge and memorize. So, we need to take this equation and plug
- 01:32
in the value of the half-life, t½ or half-life= 56.0 seconds. Solving
- 01:38
for k, we find that the rate constant equals 0.0124 per second.
- 01:42
So that means that A is the correct answer. Now quick, head over to CarbonDating.com before [Scientist using tablet]
- 01:46
you have your half-life – we mean mid-life – crisis.
Related Videos
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?
AP Chemistry 3.2 Laws of Thermodynamics. What is the value for ΔG?