ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Test Prep Videos 443 videos
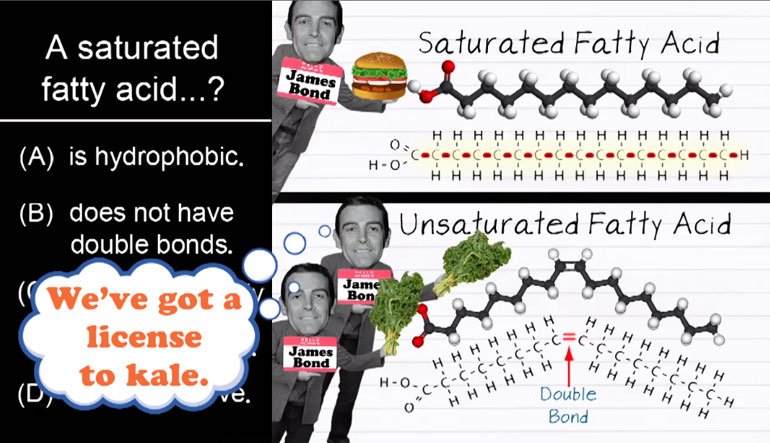
AP Biology: Biological System Interactions Drill 1, Problem 1. Complete the sentence about a saturated fatty acid.
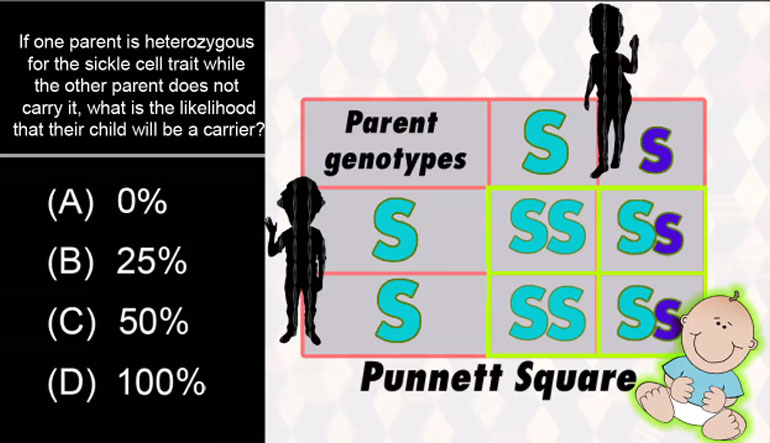
AP Biology: Essential Life Process Information Drill 1, Problem 1. If one parent is heterozygous for the sickle cell trait while the other par...
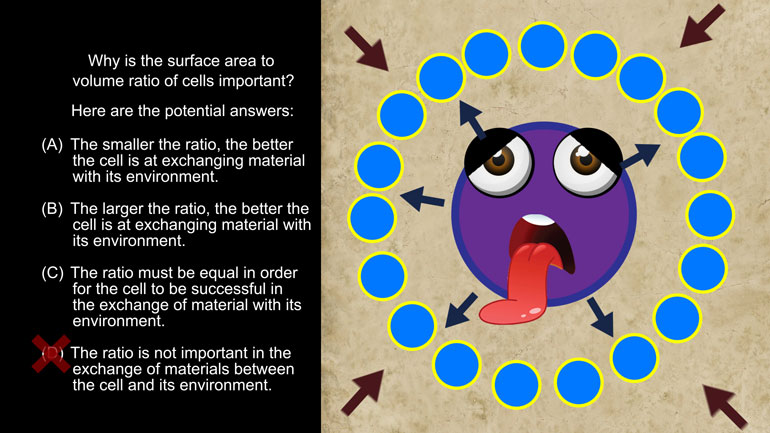
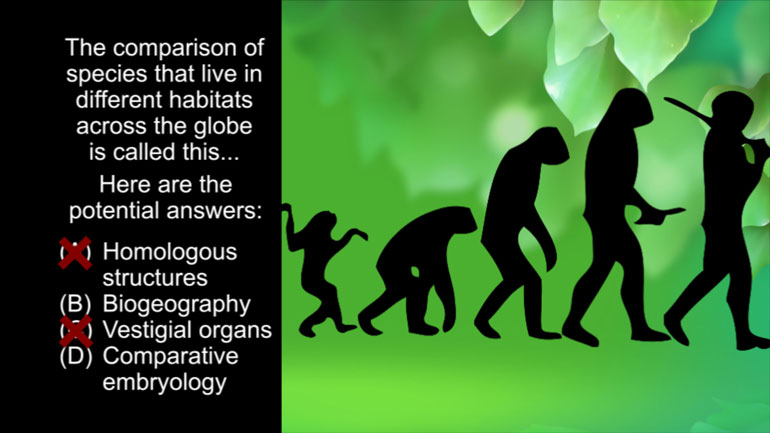
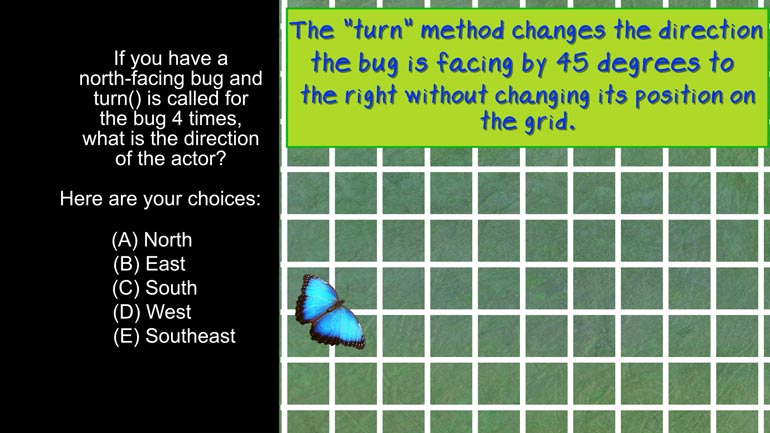
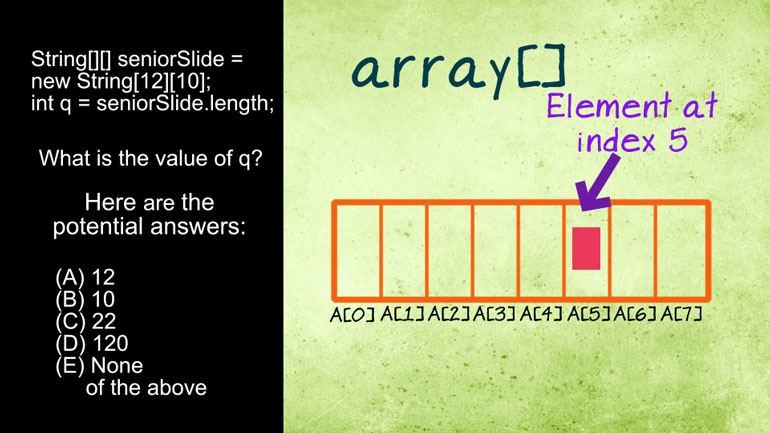
AP Biology: Evolution Drives the Diversity and Unity of Life Drill 1, Problem 1. The first cells on planet Earth were likely what?
AP Chemistry 1.3 Rearrangement and Reorganization of Atoms 11 Views
Share It!
Description:
AP Chemistry 1.3 Rearrangement and Reorganization of Atoms. How many moles of oxygen will be required for each mole of propane?
Transcript
- 00:04
Here’s your Shmoop du jour, brought to you by propane, the movement that advocates for [Window panes outside a building]
- 00:09
windows.
- 00:10
Here’s today’s question:
- 00:12
When propane, a flammable compound commonly used in gas grills, is ignited, it will react
- 00:18
with oxygen in a combustion reaction.
Full Transcript
- 00:21
The reaction can be seen below: C3H8 + O2 ? CO2 + H2O
- 00:25
Once this equation is correctly balanced, how many moles of oxygen will be required
- 00:30
for each mole of propane?
- 00:32
And here are your potential answers…
- 00:35
Okay, flex those fingers, crack those knuckles, and practice opening a jar or two, because [Man attempting to open a jar]
- 00:40
we’re gonna have to balance this whole equation by hand.
- 00:43
We’re going to release all the carbon in our propane in the form of carbon dioxide. [Carbon dioxide released from propane]
- 00:47
That means that the carbons contained in carbon dioxide should be equal to the carbons in
- 00:51
the propane.
- 00:52
So let’s balance those guys first. [Carbon molecules balancing on a scale]
- 00:53
Grab your umbrella!
- 00:54
Or whatever people use to balance on tightropes.
- 00:57
Okay, so there are three carbons on the left and one on the right, so we’ll need a coefficient
- 01:01
of 3 in front of that carbon dioxide.
- 01:04
Now we need to ask a question to our hydrogen atoms: water you doing? [Oxygen smiling with two hydrogen atoms]
- 01:09
Well, our hydrogen atoms are all busy forming water molecules when the propane is ignited…
- 01:15
Which means that we need to balance the hydrogens in propane with the hydrogens in water on
- 01:20
the other side of the equation.
- 01:22
Because for every propane, there is an equal and opposite con-pane.
- 01:25
That doesn’t mean anything. [Girl looking at a book]
- 01:26
Don’t look that up.
- 01:27
To balance our hydrogens, we observe that there are two hydrogens in water and eight
- 01:31
in propane, so it’ll take four water molecules to balance the equation.
- 01:34
But we’re not done!
- 01:36
No celebratory fist bumps just yet… [Boy offering a fist bump]
- 01:38
We still have to balance our oxygens.
- 01:40
On the right side of the equation we have 6 oxygens from carbon dioxide and 4 from water
- 01:44
for a total of 10.
- 01:46
On the left, we have two per molecule, so let’s put a five in front of that oxygen
- 01:51
to balance it out.
- 01:52
That must mean that our answer is D, since the coefficient in front of our oxygen molecule [Number 5 circled]
- 01:57
is 5.
- 01:58
Next time, maybe let’s just stick to charcoal. [Santa jumping down a chimney]
- 02:00
Or good ol’ firewood.
- 02:02
Seriously, that propane stuff was way complicated.
- 02:04
Okay, now it’s celebratory fist bump time.
- 02:07
Pound it. [Boy offering a fist bump]
Related Videos
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?
AP Chemistry 3.2 Laws of Thermodynamics. What is the value for ΔG?