ShmoopTube
Where Monty Python meets your 10th grade teacher.
Search Thousands of Shmoop Videos
Test Prep Videos 443 videos
ACT Science: Research Summary Passage Drill 2, Problem 1. Why do you think that the filter paper will not remove the salt from the water?
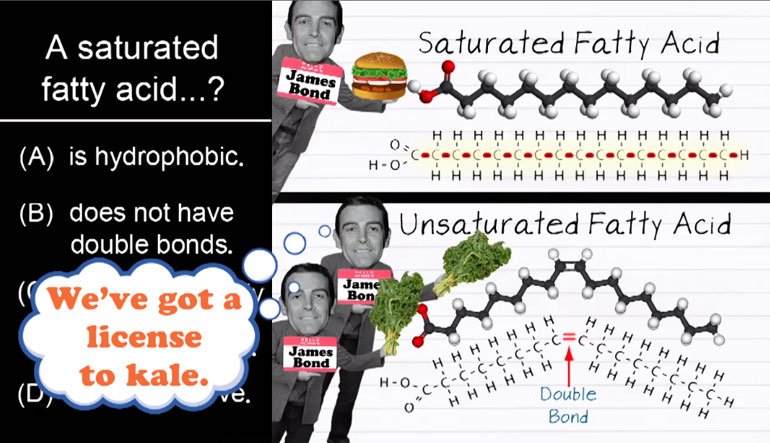
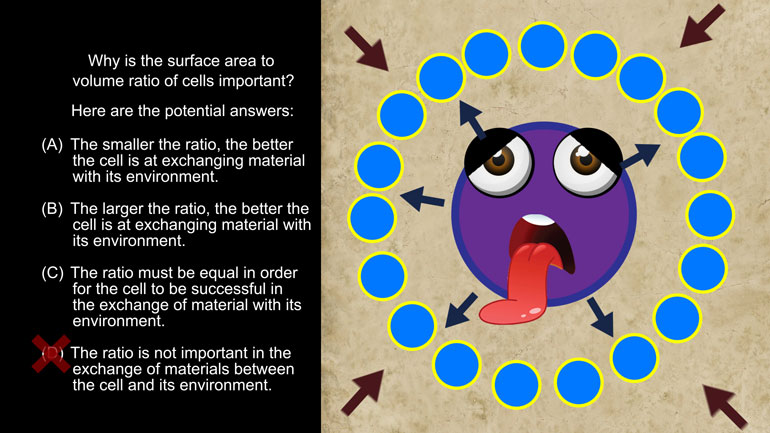
AP Biology: Biological System Interactions Drill 1, Problem 1. Complete the sentence about a saturated fatty acid.
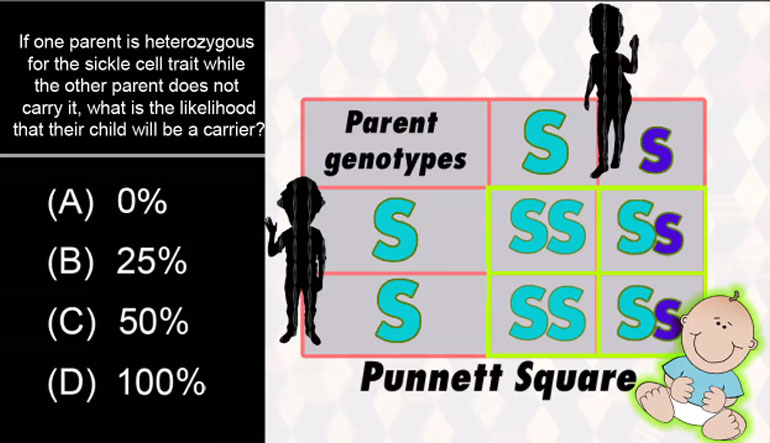
AP Biology: Essential Life Process Information Drill 1, Problem 1. If one parent is heterozygous for the sickle cell trait while the other par...
AP Chemistry 1.2 Chemical Reaction Rates 8 Views
Share It!
Description:
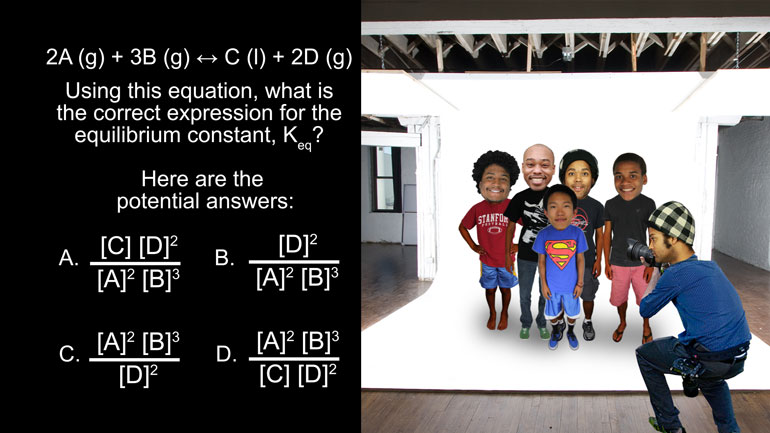
AP Chemistry 1.2 Chemical Reaction Rates. Approximate the appearance rate of ozone.
Transcript
- 00:04
And here’s your Shmoop du jour, brought to you by oxygen. [Man on a sofa surrounded by oxygen]
- 00:07
We love it so much we’d die without it.
- 00:10
…Literally.
- 00:11
Okay, here’s our question: The disappearance rate of oxygen in the reaction
- 00:16
3 O2?
Full Transcript
- 00:17
yields 2 O 3 is equal to 0.40 moles per liter per second.
- 00:24
Approximate the appearance rate of ozone.
- 00:26
And here are your potential answers…
- 00:28
All right, so we know the rate at which O2 is disappearing. [Magician makes O2 disappear]
- 00:34
All we need to do is use the stoichiometry of the reaction equation
- 00:39
to find the rate of O3 appearance.
- 00:42
Easy peasy lemon squeezy? [Lemon squeezed on equation]
- 00:43
Alright, well it's not as scary as it sounds…
- 00:46
Using the reaction equation, for every 3 oxygen molecules that disappear, 2 ozone molecules
- 00:52
appear.
- 00:53
O2 is disappearing at a rate of 0.40 moles per liter per second.
- 00:57
No, not that kind of mole – the Avogadro’s number kind. [Mole in the ground and Avogadro's constant lands on its head]
- 01:00
Yeah, it’s confusing.
- 01:01
Just don't try shoving a mole into a beaker and you should be okay. [Student holding a mole in a beaker]
- 01:05
Anyway, we can set up a proportion using the stoichiometry and reaction rates to find x,
- 01:10
which represents the unknown rate of O3 appearance.
- 01:13
Which is what the question is asking for, in case you got lost over there in the labyrinth [A couple lost in a maze]
- 01:17
of chemistry and angry moles…
- 01:20
If we multiply both sides of this equation by 0.4 moles per liter per second and crunch
- 01:25
the numbers…
- 01:26
Mm, mm…
- 01:27
Mathy…
- 01:28
… We find out that the rate of appearance of ozone is 0.27 moles per liter per second.
- 01:35
So B is the correct answer.
- 01:37
Now go take a deep breath and thank a tree for that sweet, sweet O2 . [Girl takes deep breath and hugs a tree]
- 01:39
Seriously, write it a thank you card.
- 01:42
No one ever appreciates all the hard work its doing…[Thank you card by a tree]
Related Videos
AP Chemistry 1.3 Chemical Reaction Rates. What is the overall order of the reaction?
AP Chemistry 1.4 Chemical Reaction Rates. What are the correct units for a second order rate constant?
AP Chemistry 1.5 Chemical Reaction Rates. What is the rate law for the reaction?
AP Chemistry 3.2 Laws of Thermodynamics. What is the value for ΔG?